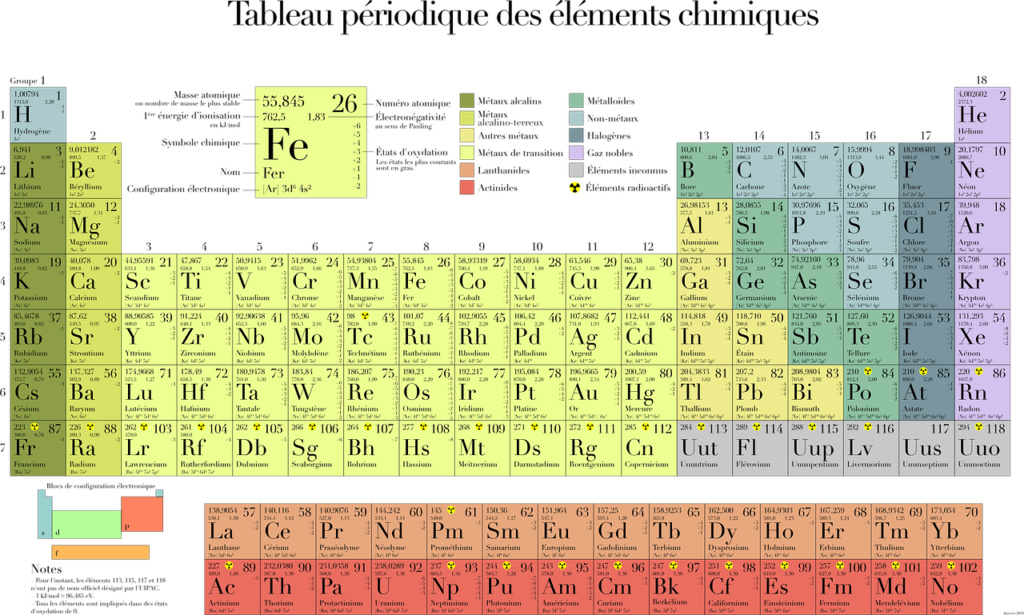
1. Periodic Properties and Variations of Properties – Physical and Chemical
(i) Periodic properties and their variations in groups and periods.
(ii) Periodicity on the basis of atomic number for elements.
2. Chemical Bonding
Electrovalent, covalent and co-ordinate bonding, structures of various compounds – orbit structure and electron dot structure.
(a) Electrovalent Bonding
(b) Covalent Bonding
(c) Coordinate Bonding
3. Study of Acids, Bases and Salts
(i) Simple definitions in terms of the molecules and their characteristic properties.
(ii) Ions present in mineral acids, alkalis and salts and their solutions; use of litmus and pH paper to test for acidity and alkalinity.
(iii) Definition of salt; types of salts.
(iv) Action of dilute acids on salts.
(v) Methods of preparation of Normal salts with relevant equations. (Details of apparatus or procedures not required).
4. Analytical Chemistry
(i) Action of Ammonium Hydroxide and Sodium Hydroxide on solution of salts: colour of salt and its solution; formation and colour of hydroxide precipitated for solutions of salts of Ca, Fe, Cu, Zn and Pb; special action of ammonium hydroxide on solutions of copper salt and sodium hydroxide on ammonium salts.
(ii) Action of alkalis (NaOH, KOH) on certain metals, their oxides and hydroxides.
5. Mole Concept and Stoichiometry
(i) Gay Lussac’s Law of Combining Volumes; Avogadro’s Law.
(ii) Refer to the atomicity of hydrogen, oxygen, nitrogen and chlorine (proof not required).
(iii) Vapour Density and its relation to relative molecular mass.
(iv) Mole and its relation to mass.
(v) Simple calculations based on chemical equations.
6. Electrolysis
(i) Electrolytes and non-electrolytes.
(ii) Substances containing molecules only, ions only, both molecules and ions.
(iii) Definition and explanation of electrolysis, electrolyte, electrode, anode, cathode, anion, cation, oxidation and reduction (on the basis of loss and gain of electrons).
(iv) An elementary study of the migration of ions, with reference to the factors influencing selective discharge of ions, illustrated by the electrolysis of: molten lead bromide; acidified water with platinum electrodes; aqueous copper (II) sulphate with copper electrodes; electron transfer at the electrodes.
(v) Applications of electrolysis.
7. Metallurgy
(i) Occurrence of metals in nature.
(ii) Stages involved in the extraction of metals.
(iii) Extraction of Aluminium.
(iv) Alloys – composition and uses.
8. Study of Compounds
A. Hydrogen Chloride
Hydrogen chloride: preparation of hydrogen chloride from sodium chloride; refer to the density and solubility of hydrogen chloride (fountain experiment); reaction with ammonia; acidic properties of its solution.
B. Ammonia
Ammonia: its laboratory preparation from ammonium chloride and collection; ammonia from nitrides like Mg3N2 and AlN and ammonium salts. Manufacture by Haber’s Process; density and solubility of ammonia (fountain experiment); aqueous solution of ammonia; its reactions with hydrogen chloride and with hot copper (II) oxide and chlorine; the burning of ammonia in oxygen; uses of ammonia.
C. Nitric Acid
Nitric Acid: one laboratory method of preparation of nitric acid from potassium nitrate or sodium nitrate. Large scale preparation. Nitric acid as an oxidizing agent.
D. Sulphuric Acid
Large scale preparation, its behaviour as an acid when dilute, as an oxidizing agent when concentrated – oxidation of carbon and sulphur; as a dehydrating agent – dehydration of sugar and copper (II) sulphate crystals; its non-volatile nature.
9. Organic Chemistry
(i) Introduction to Organic compounds.
(ii) Structure and Isomerism.
(iii) Homologous series – characteristics with examples.
(iv) Simple nomenclature.
Simple nomenclature – of the hydrocarbons with simple functional groups – (double bond, triple bond, alcoholic, ether, aldehydic, keto, carboxylic group) longest chain rule and smallest number for functional groups rule – trivial and IUPAC names.
(v) Hydrocarbons: alkanes, alkenes, alkynes.
(vi) Alcohols: ethanol – preparation, properties and uses.
(vii) Carboxylic acids (aliphatic – mono carboxylic acid): Acetic acid – preparation, properties and uses of acetic acid.